Table of Contents
In the wake of the COVID-19 pandemic, the medical supply industry saw an alarming rise in counterfeit CE certificates, particularly for essential items like surgical gowns. This guide aims to equip our readers with the knowledge and tools needed to authenticate CE certifications of disposable sterile surgical gown factory. As European Union regulations and standards play a crucial role in ensuring product safety and quality, verifying CE certification involves a multi-step process. Our article will walk you through these steps, helping you navigate the complex world of medical device certification and safeguarding your supply chain against fraudulent claims. Whether you’re a healthcare professional, a procurement specialist, or simply a concerned individual, this guide will provide valuable insights into maintaining the integrity of surgical gown sourcing in these challenging times.

Verifying the CE mark on surgical gowns is crucial for ensuring compliance with EU directives. This mark must be visible, legible, and indelible on the product itself, prominently displayed on the packaging, and included in all accompanying documentation. This comprehensive approach to CE marking not only meets regulatory requirements but also instills confidence in healthcare professionals and distributors about the product’s safety and reliability. For surgical gown factory, meticulous attention to proper CE marking is essential to avoid potential regulatory issues and maintain market credibility.

When it comes to Class I sterile surgical gowns, quality and certification are pivotal. So, did you know that the CE mark on these gowns must be accompanied by a four-digit Notified Body number? This isn’t just a random number; it signifies the involvement of a Notified Body in the rigorous certification process.
Check the Declaration of Conformity (DoC) of the Surgical Gown Factory

When verifying CE certification of surgical gown factories, the Declaration of Conformity (DoC) is a crucial document to examine. This official statement from the manufacturer confirms that their products meet EU directives and regulations, serving as a roadmap for compliance.
A valid DoC should include several key elements: the manufacturer’s full name and address for accountability, a detailed product description with a unique identifier, an explicit statement of conformity to relevant EU directives, and a list of applicable harmonized standards. These components provide a comprehensive overview of the product’s compliance status.
Finally, the DoC must be signed by a responsible person and include the date of issue. This signature adds a layer of authority and trust, indicating that someone is personally vouching for the product’s compliance. By thoroughly reviewing these aspects of the DoC, you can ensure that the surgical gowns meet the necessary standards and regulations.
Confirm Notified Body Involvement of the surgical gown factory
When verifying CE certification of surgical gown factory, identifying the Notified Body is crucial. Look for the four-digit number next to the CE mark on the gown’s label. This number is your key to confirming compliance and the involvement of a legitimate Notified Body in the certification process.
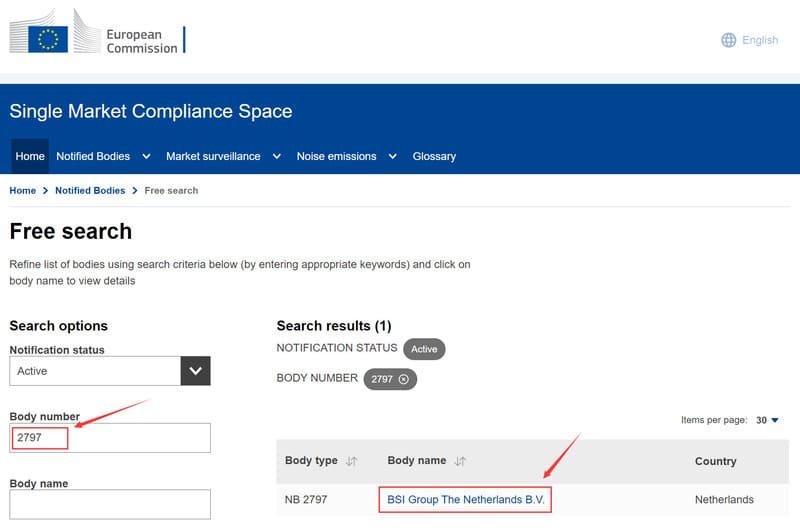
To verify the Notified Body’s authorization, use the NANDO (New Approach Notified and Designated Organisations) database. Enter the four-digit code into this official resource to confirm if the Notified Body is authorized for the specific certification. Relying solely on the NANDO database ensures accuracy and helps maintain regulatory compliance, assuring customers about the quality and legitimacy of the surgical gowns.
Review Compliance with Relevant Standards of sterile surgical gown
For surgical gown manufacturers, compliance with key industry standards is essential.
EN 13795 ensures that gowns provide adequate barriers against fluids and contaminants, crucial for infection prevention during surgeries. Equally important is EN ISO 11607, which governs the packaging of sterilized medical devices, ensuring gowns remain sterile until use.
Adhering to these standards not only meets regulatory requirements but also builds trust with healthcare providers, demonstrating a commitment to quality and patient safety in the production of surgical gowns.
Examine the Sterilization Information of the CE-certified surgical gown
Sterilization Methods
Surgical gown factories primarily use two sterilization methods:
Ethylene Oxide (EtO): Effective for medical textiles, EtO gas eliminates microorganisms but requires careful handling due to toxicity.
Gamma Irradiation: Uses ionizing radiation for bulk sterilization, penetrates complex materials, and leaves no residue.
Sterilization Validation
Validation is crucial and governed by specific standards:
ISO 11135: Pertains to ethylene oxide sterilization. It establishes requirements and provides detailed guidelines to ensure that the EtO process is both effective and safe. Compliance ensures that every product leaving the surgical gown factory is free from contaminants.
ISO 11137: Focuses on ensuring the effectiveness of gamma irradiation. This standard outlines the necessary steps for validating the gamma sterilization process, ensuring consistent and reliable results.
Adherence to these methods and standards ensures surgical gowns meet the highest safety criteria for surgeon use.
Review Technical Documentation from the Surgical Gown Factory
The technical file is crucial for verifying the quality and safety of surgical gowns from top manufacturers.
Product Description and Specifications
The technical file includes detailed product descriptions and specifications, akin to a cookbook for surgical gowns. It outlines materials used, design specifics, and manufacturing processes, providing comprehensive information about the product’s composition and construction.
Test Reports: Proof of Compliance
Test reports in the technical file demonstrate compliance with essential requirements. These documents prove that each gown has passed rigorous testing. For example, ASTM International standards require surgical gowns to resist liquid penetration at varying levels, from 100 cm H2O for Level 1 to 300 cm H2O for Level 4 gowns. These reports ensure the gowns meet necessary quality and safety standards.
In summary, verifying CE certification of surgical gown factories involves checking the CE mark format, reviewing the Declaration of Conformity, confirming Notified Body involvement, ensuring compliance with relevant standards, examining sterilization information, and reviewing technical documentation. These steps are crucial for ensuring the quality, safety, and regulatory compliance of surgical gowns.
Need assistance with CE certification verification or sourcing high-quality sterile surgical gowns? Contact Morntrip for expert guidance and support. Our team can help streamline your verification process and connect you with reliable surgical gown manufacturers that meet stringent CE certification requirements.






